** Reading the Manual on phones and smaller screen tablets is not recommended, since you may not enjoy the best user experience.
Alchemy’s Laboratory Information Management System (LIMS) is designed to embed testing right into the development process, bringing in real-time data from lab equipment and ensuring quality at every step. The Quality Control process provides a ready-to-use library of test methods, analyses, and charting tools. Moreover, it bridges the gap between formulation and testing with a seamless integration while removing silos between departments.
This manual will help you navigate the Quality Control process within Alchemy while learning best practices for the product.
In order to begin a Quality Control process, two independent records must be created:
Once the Material record has been created it can be used in the QC specification record to provide testing parameters and other relevant information to the Quality Control process.
The Quality Control process is comprised of three records:
The Material record is a data structure used to store information about raw materials that may be used in an organization's product R&D and manufacturing.
The record contains the following General Information fields:

In addition to seeing current and historical samples of the material record, this drawer can be used to create new samples. After opening the Sample drawer, users can click + New Sample in the top right corner. This action opens a new Sample record that can be completed. Once all required fields have been filled in, the record can be closed and upon refresh of the material record Sample drawer, the newly created sample will appear.
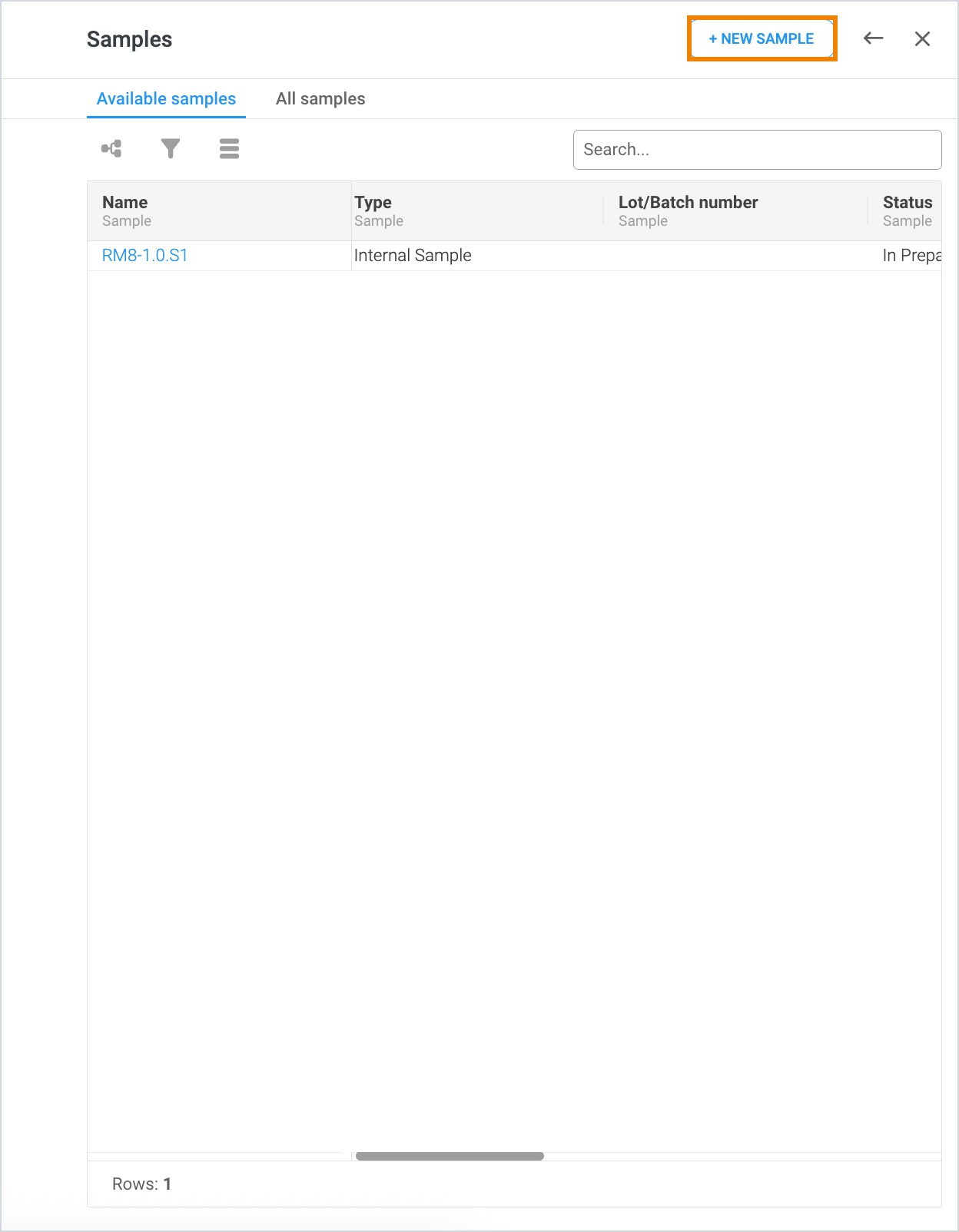
A new material version can be created by clicking + New Version within the Material Versions drawer. This action opens a new Material record as a duplicate of the original material. Version numbering would increase from 1.0 to 2.0 when creating a new version of the material.

The naming pattern generated in the Code field is based on the value selected in the Category field, found under the Categorization section.
In the event the Category field has not been set, the generated code will appear with the naming pattern used for Other.
The Categorization section of the Material record includes:

The Suppliers section of the Material record includes:

The Documents section of the Material record includes:

The Chemical Information section of the Material record includes:

The Hazard and Safety section of the Material record displays the GHS pictograms related to the material:

The 1:1 Replacements section of the Material record displays a table used to list any applicable replacements.

The Specification section of the Material record displays a list of all properties associated with the material’s type or subtype. The table will only be visible if the fields have been selected and there are properties associated with them.
Targets and Value for calc. can be defined for all specifications. Values will be used in all calculations and color-coded based on the given targets.

The Recipe section of the Material record includes a complete set of formulating tools to develop a recipe and capture all relevant property data. Materials, phases, processing steps, and comments can be added using the buttons below the table.

The QC Specification record is used to inform a user how samples of a material are meant to be tested in the Quality Control process.
The record contains the following General Information fields:

Note: Only one material can be selected per QC Specification record. However, multiple versions of a specific material can be added. Newly created versions of the QC Specification can only be linked to the same material and its versions.
⚠️ When a QC Specification is created, it must be published by clicking the Publish button to use it across the system. Non-published, or Draft, specifications are not included in data models and are unable to be used in the Quality Control process. Brand new QC Specification can only be linked to a Material which does not already have a QC Specification linked.
If any fields are manually added to the record via configuration, then they will default to only being editable when a new version of the QC Specification is created. To change this and make the fields editable at all times, an update to the OnChange event of the QC Spec Status field needs to occur. When manually adding the field, add lines for
.Editable = false
.InputBackgroundColor = ‘#F5F7FA’
See the image below for an example. Talk to your System Administrator for any Configuration Changes.

A new QC Specification version can be created by clicking New Version to the right of the record version number or by clicking + New Version in the QC Specification version drawer. This action opens a new QC Specification record as a duplicate of the original record. Version numbering would increase from 1.0 to 2.0 when creating a new version of the QC Specification.

The Preparation Work section of the record includes information for the user related to materials needed or tasks to complete prior to testing being performed. Fields in this section include:
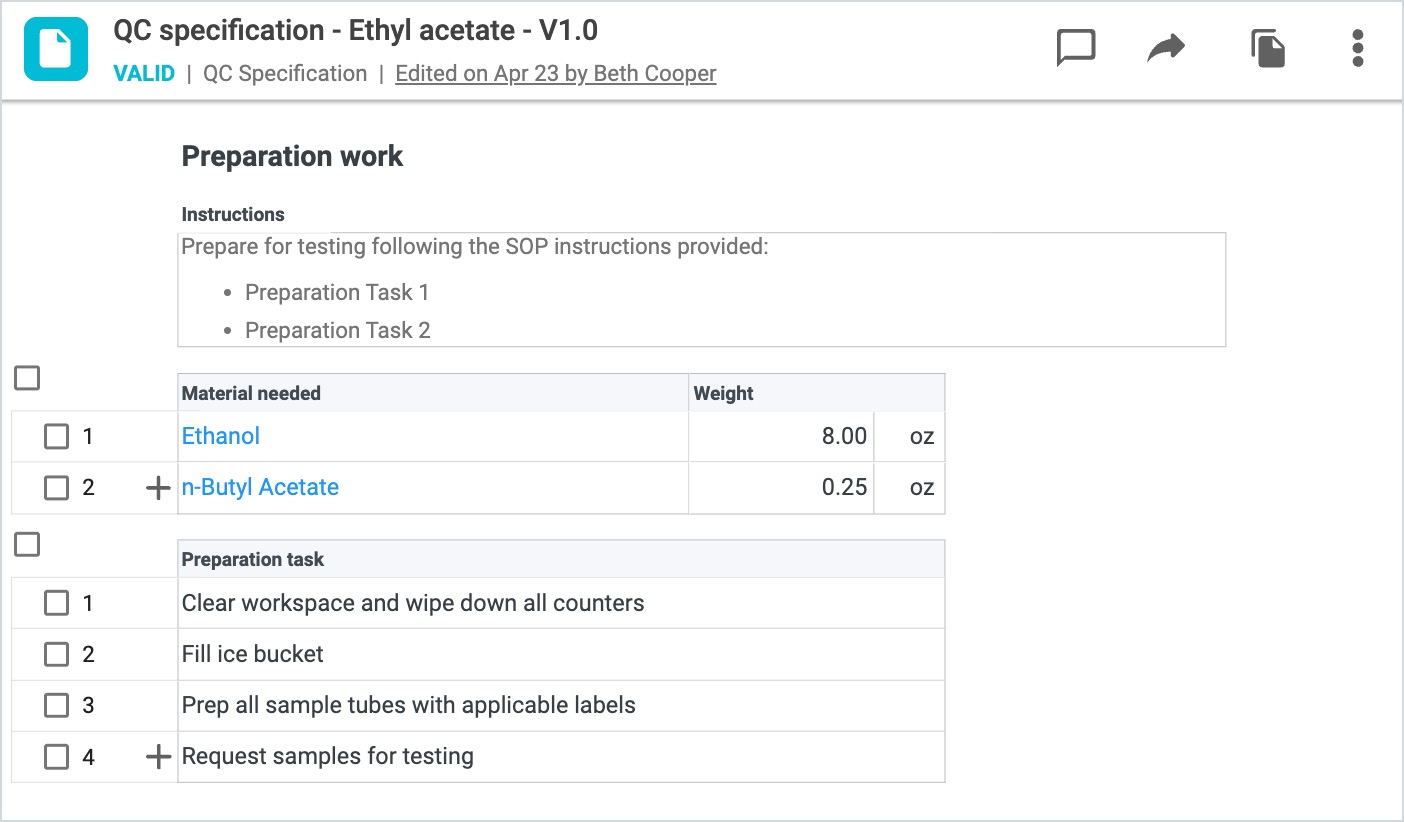
The Post Testing Work section of the record includes information for the user related to tasks to complete once testing has ended. Fields in this section include:

The QC Testing Specification/Process Flow section of the record is used to display selected tests and test methods the user should perform on samples. These tests can be grouped into stages which are added by clicking the Add Testing Stage button.

Each stage is displayed as a table with the following fields:
Tests and Test Methods are added to the Specifications table to define the parameters a user will reference when completing sample testing in a Quality Control process. They can be added to the table by clicking:
To add tests through the modal, measured properties, as well as the applicable units, must be defined. The user can select one or more properties via the dropdown, which will display a list of all available system properties measured in the lab. Additional properties can be added by clicking +Add.
Based on the selected properties, one of two additional sections will appear:
In the Conditions section, the user can choose optional conditions for this test, including its number of variations. However, mandatory conditions will be added automatically. For numerical conditions, the user can select whether they want to enter values manually or if they would like to define a range. In this case, the system will generate all values. Additional conditions can be added by clicking + Condition.
In the Specify Condition Values, a table will be auto-generated based on the selected properties and conditions chosen. Properties will be added as new rows, while conditions will display as columns. The system will multiply the number of rows for each varying condition to match the number of variations for the associated property.

When saved, the system will add all necessary rows to the Tests table with all user-defined properties, and their respective conditions.
Test Methods (Strict)
Test Methods (Flexible)
Single Test
Quality Control is a process template representing Alchemy’s LIMS platform, with only a single stage.
Pre-configured records found in this stage include:
Additional records can be defined in the configuration portal and added to the Quality Control process template based on organizational needs.
When a user initiates the creation of a Quality Control process, the QC Overview and Preparation record is automatically generated and displayed in the process navigation panel to the left of the open record.
This record is comprised of four sections and is primarily auto-populated with information from the QC Specification record once a Material is selected:
*Note: A new instance of this record will only have the General Information section displayed. Additional sections will not appear until the Material has been selected and applied to the field.
The General Information section of the record includes:

The Preparation Work section of the record includes:

The Post Testing Work section of the record includes:

The QC Testing Specification/Process Flow section of the record includes stage table(s) with the following non-editable fields:

Once the QC Overview and Preparation record is in a valid state and samples have been received, the user is ready to begin testing and the Ready For Sample Testing button becomes active. Once the button is clicked, the QC Sample Testing record will be created and testing of samples can begin.
The QC Sample Testing record has two main functions:
In certain cases, a sample may fall out of specification based on the guidelines set prior to testing. If this happens, the QC Sample Testing record will be used to create adjustments to the initial recipe in the Trials table.

The Trials table displays the following default columns and information:
Additional Material data and columns can be added to the Trials table through the Settings icon and include:
Materials and Material Properties columns will remain visible even when horizontal scrolling is required to view additional adjustment columns.
Add a new row to the Formulations table by clicking the options directly beneath the table:
Hovering over a row within the table displays actionable options icons on the left-hand side:
Rows added via the options displayed beneath the Trials table will appear at the bottom of the table. Rows added via the + icon will appear directly beneath the row that was selected.
Along with these adjustments, calculations of properties with defined targets will be run on the newly created samples and will be captured in the Calculations table. Additional calculations can be run by adding them with the + Calculation button beneath the table. This will create a row for data capture.

Sample testing occurs in the Tests table at the bottom of the QC Sample Testing record. Tests are performed in order of appearance in the table. As tests are completed and data is entered into the table, the cells will turn either green (within specification) or red (out of specification).
Certain properties can be marked as Include in Certificate of Analysis and/or Stop of Failure.
If a property is to be included in the CoA and the test passes the required targets, a checkmark icon will be displayed in the cell signifying this value will be entered in the certificate. If multiple tested Samples fit within required targets, the newest value will be automatically marked, while users retain the option to manually choose which passing value will be added to the CoA.
If a property is marked as Stop of Failure and the test does not pass the required targets, an exclamation mark icon will be displayed in the cell and the entire testing table will become non-editable, as further testing on this Sample should not be performed. In this case the user needs to stop testing and proceed with one of possible options below.

If all sample testing results are within the predefined targets, the Sample Approved button will become active. However, if additional testing is required because a value returned out of range, additional buttons will become visible.
The + Adjustment button will add a column to the Trials table above.

Figure 3.8 Adjustment Column - Trials Table
Additional adjustment columns can be added to the Trials table by creating and applying properties for:
The use of these columns will help show the increase in weight in relation to the previous amount so users can track the impact of an adjustment better over time.
See Appendix B for more information about how to set up the adjustment properties in the system.
Once the formulation is complete and adjustments are made, the user will click the Send Adjustment button at the bottom of the record that will become visible at this time. After the new sample has been prepared with the adjusted values, it will undergo the testing process in the Tests table again.
Users also have the option to Ask For Assistance from a lab manager or mark a sample test as Out Of Specification. This option lets the lab manager decide how the user should proceed with that sample and any further testing.
The Ask For Assistance feature creates a log where the user can create an entry of the solution to their problem, as well as who they contacted for help. While they receive help, the job status of the process will be updated to Waiting for assistance until the issue has been resolved.

In the event a solution cannot be reached, the sample can be marked as Out of Specification. At this point, the lab manager will decide whether to request a customer sample, continue with formulation and adjust the sample for further testing, repeat testing on the same sample or discard the testing entirely by marking it Off-Grade.

Once testing has been completed and the Sample Approved button has been clicked, the Post Testing Work record will be generated.
The Post Testing Work record includes information for the user related to tasks to complete once testing has ended. It is comprised of three sections:
The Requirements section of the record has fields for:

The Samples section contains a table with the following information:

Users can also request new samples with the + Request Sample button. This will display a section where users can input the:

The Conclusion section of the record contains a rich text field where users can enter comments about testing for future jobs. If the sample type is a customer sample, there will be an additional field for any customer comments.

A variety of integrated record templates exist to support Alchemy’s Quality Control and can be categorized into the following groupings:
Users can create new records by clicking + New in the left-hand navigation panel. This opens a drawdown menu where the necessary record can be selected from the list. A modal will open, allowing the user to input the values required to save and create the record.
Each record contains a General Information section that is used to document relevant high level information. Additional record-specific sections may follow. Details of each record template can be found in the following subsections.
Note: The creation and use of these record is dependant upon the user having the appropriate permissions. Contact the system administrator if there are access issues.
However, the metadata for these record templates cannot be configured, regardless of permissions.
Testing records relevant to the Quality Control process include:
The Sample record is a data structure used to store information about physical substances that undergo testing to discover their properties.
Within Alchemy, Sample records are used to test materials through the Quality Control process.
The record contains the following General Information fields:

Figure 4.1 Sample - General Information
Note: The sample status automatically changes to Opened when a sample is consumed. When the available quantity of the sample becomes zero or below, the status is automatically updated to Not Available. All other status changes are done manually
The Availability and Usage section of the Sample record includes:

Figure 4.2 Sample - Availability and Usage
The Sample Location section of the Sample record includes:

Figure 4.3 Sample - Location
The Hazard and Safety section of the Sample record displays the GHS pictograms related to the material. This information is pulled directly from the origin material and is uneditable from the Sample record.
This section will not be visible until a Material has been associated with the sample.

Figure 4.4 Sample - Hazard and Safety
The Sample Label record is used to generate a printed label to attach to a sample container.
It can be created by clicking the Print Label button from the Sample record. Once a label for a sample is made, additional clicks will display the same label as what was previously printed.

Figure 4.5 Sample Label
Every Sample Label record has the following information:
The Chemical record is a data structure used to store information about substances an organization is using.

Figure 4.6 Chemical
A new Chemical record has the following fields:
INCI, also known as International Nomenclature Cosmetic Ingredient, is a database of internationally recognized names of cosmetic ingredients. Use of the INCI Name record will enable a user to link chemicals used to any applicable INCI Names, maintaining the connection to its international naming conventions while being able to utilize organization identifiers when running trials.

Figure 4.7 INCI Name
A new INCI Name record has a single field:
Specification records relevant to the Quality Control process include:
Conditions can be used in Alchemy to describe the circumstances under which something is measured, such as the temperature and humidity for a dry time measurement or time interval testing of bacterial growth.

Figure 4.8 Condition
Much like Properties, conditions can be added at any point in the process. Once applied, Alchemy automatically expands your data model and metadata, and propagates the addition(s) to the internal library of conditions. Once a condition is defined, it becomes available for use by users with the appropriate permissions within:
🔐 Conditions can be applied only to the production tenant, not the UAT.
⚠️ When a condition is created, it must be applied by clicking the Apply button to use it across the system. Non-applied, or Draft, conditions are not included in data models, and metadata cannot be specified as a target or be measured.
The Condition record contains the following General Information fields:
Note: For additional information regarding types supported by Alchemy, please refer to the section Property - Property Types.
Condition records will also display:
In Alchemy, a property can be used to characterize many elements in the system including:
Properties can be added to the system at any point in the process. Once applied, Alchemy automatically expands your data model and metadata, and propagates the addition(s) to the internal library of properties. Once a property is defined, it becomes available for use within the system by users with the appropriate permissions:
🔐 Properties can be applied only to the production tenant, not the UAT.
The Property record contains the following General Information fields:

Figure 4.9 Property - General Information
⚠️ When a property is created, it must be applied by clicking the Apply Property button to use it across the system. Non-applied, or Draft, properties are not included in data models, and metadata cannot be specified as a target or be measured.
This type of usage allows for property values to be manually entered into the system, or it can be calculated based on other measured properties. Once applied, this property is available for use in Tests tables across the system.
Selection of this usage will trigger an expression field to define any applicable:
Two additional sections will also become visible at the bottom of the Property record if this usage is selected:

Figure 4.10 Property - Measured in Lab tests
This type of usage calculates properties with a formula and can be based on other properties or information from the Trials table. However, with the appropriate configuration this property usage can also be defined to allow for the value to be editable inside the Calculations table. Once applied, this property is available for use in Calculations tables across the system.
Selection of this usage will trigger two additional expression fields to define any applicable:

Figure 4.11 Property - Calculated
There are eight property types available within Alchemy. Depending on the type selected, additional fields will be added to the record. These fields are outlined below.
Unit Type & Default Unit: Supported unit types, as well as with applicable units, can be found in Appendix 1 - Units Library.
Predefined Values: If enabled, a field appears where a comma separated list of predefined numerical values can be entered for the property.
Applied properties will then be displayed as a dropdown with a list of these values.
Decimal Places: Used to define the desired number of decimal places for a displayed property value across the system.
The default value is two decimals.
Scientific Notation: Used to define whether a property value should be displayed in scientific notation.
Use Property for Formulating: Used to enable a property to be selected as the primary formulating input within a lab book.
Selection of this option will trigger an expression field to define the calculation of the weight for each material based on the entered value:
If enabled, the system will automatically select the option Use property for Compare Material Contribution while formulating. This is due to Scale functionality. The system needs both expressions to be able to recalculate weights.
Predefined Values: Numerical and text values only.
Additional information regarding this option can be found in the Property Types, Number section.
Date & Time Format: Used to define the format that will be used whenever the property is displayed in the system. The default format is:
Allow multiple: If enabled, multiple attachments are able to be added to this property type in the system.
Use Property for Formulating: Additional information can be found in the Property Types, Number section.
Allow multiple: If enabled, multiple users are able to be added to this property type in the system.
No additional field is displayed in the property record. If this type is selected, a field will be displayed for the property value which will require the entry of a valid url format.
A record reference property type allows the user to define the criteria around which record templates, as well as any desired columns, are visible to a user when the property is used in a lab book.

Figure 4.12 Property Type - Record Reference
Within the Property record, one or more record templates can be selected. Filter criteria can be applied for each record template listed so only the names of records with corresponding templates and criteria will be visible to the user interacting with the field and selecting the property value.
Once a value has been selected from the dropdown, columns visible from the referenced record(s) will be displayed. Visible columns can include:
Note: To enable a property or condition to be used as a record reference, the templates must be appropriately configured in the configuration portal. Contact your administrator or Alchemy representative for any assistance with enabling this feature.
Once all required fields are complete, the property can be applied to and used in the system. If changes need to be made to a property, certain fields can be updated including:
Any changes made to these fields or selections will enable the Apply Changes button and issue a cation that the property contains unsaved changes. Once clicked, all changes will be saved and displayed in the system.
Fields that cannot be updated once a property has been applied to the system include:

Figure 4.13 Property - Apply Changes
If an applied property is no longer needed, it can be archived in the system to prevent it from being used further. Clicking the Archive Property button will allow for the system to retain all historical trials or test methods the property was used in. While this action removes the property from use in lab books or test methods, the record can still be accessed and unarchived for future use by clicking the Unarchive Property button.
Test Methods are used in Alchemy to define any associated conditions, which are then used to perform defined trials in a Lab Book.
Test Methods can be added at any point in the process by clicking + New in the blue navigation bar. Once added, Alchemy automatically expands your data model and metadata, and propagates the addition(s) to the internal library of test methods. Once a test method is defined, it becomes available for use by users with the appropriate permissions within:
The General Information section of the Test Method record includes:

Figure 4.14 Test Method - General Information
The Requirements and Procedure section of the record is used to define all related Requirements and Procedure Descriptions in free-text entry fields.

Figure 4.15 Test Method - Requirements and Procedure
The Tests section of the record is used to select measured properties from the system library of properties marked as Measured in Lab Tests. Each property selected will display a table for capturing measurements.

Figure 4.16 Test Method - Tests
The columns for each property table include:
New rows can also be added to the property tables for users to:
Note: Each property table needs at least one row before the Test Method can be used in a Lab Book.
Material Type records are used to categorize materials in the system and can be created at any point in the process by a user with the appropriate permissions.
The General Information section of the record consists of the Material Type Name.
The Subtypes section of the record allows users to associate all applicable subtypes that are for the material type.
Note: Subtypes can only be associated with one material at a time.
The Specifications section of the record allows users to list properties associated with the material type. Selected properties will then display on all material records marked as the corresponding type.

Figure 4.17 Material Type
Material Type records are used to categorize materials in the system and can be created at any point in the process by a user with the appropriate permissions.
The General Information section of the record consists of the Material Subtype Name.
The Belongs To section of the record allows users to all material types the subtype is associated with.
The Specifications section of the record allows users to list properties associated with the material subtype. Selected properties will then display on all material records marked as the corresponding subtype.

Figure 4.18 Material Subtype
The Organizational records relevant to the Lab Book include:
The Location record is used to define storage information and conditions of items for an organization or department.
The General Information section of the record includes:
In the Sublocation section, multiple sublocations can be associated with a Location record.

Figure 4.19 Location
The Company record is used to define relevant information related to companies an organization may interact with. Interactions may include:

Figure 4.20 Company - General Information
The General Information section of the record includes:

Figure 4.21 Company - Additional Details
The Location List section allows users to create as many new locations as needed to associate with the company.
The Contacts List section allows users to create as many new contacts as needed to associate with the company.
The Subsidiaries section allows users to create as many new subsidiary companies as needed to associate with the company.
The Contact record is used to store information relevant to an organization's point of contacts for various companies they interact with.

Figure 4.22 Contact
The General Information section of the record includes:
Address, City, and Country pulled from the selected location
The table displays all system supported auto-conversions between units of the same type.
Note: The appropriate permissions are required to make and apply properties to the system. Contact your system administrator for any questions.
As stated in Section 3.2.2 - Sample Testing, additional adjustment properties can be added as columns to the Trials table. To make and display these columns, use the following steps:
Create a new property record for the column, Adjustment %. Use the images below to fill out the record details.
Once the record is complete, click Apply Property to make the property visible to the system.


Figure 6.1b Adjustment Percentage
Next, create and apply the property record for Adjustment Estimate. Both columns should appear in the Trials table when an adjustment needs to be made in the Sample Testing record. Once the record is complete, click Apply Property to make the property visible to the system.
Both columns should now appear in the Trials table when an adjustment is added to the Sample Testing record.

In both our user manual and API documentation, you will find comprehensive instructions on how to use our API for downloading your data. Our API is designed to give you access to all your personal data that we have stored, encompassing both the information you have provided and the data generated during your use of our services. By following these guidelines, you will be able to effectively retrieve your information during or at the end of your relationship with Alchemy.
Additionally, it is important to note that when you decide to end your association with Alchemy Cloud, we ensure that all of your data is thoroughly deleted from our systems. This step is crucial in making certain that Alchemy Cloud retains no access to your data once your service has concluded.